Universal ChemicalsManufactures of
|
|||||||||
|
|||||||
![]()
Interpretation
of reaction of Alum and Sodium Aluminate in Water Treatment.
-
to produce polyaluminium hydroxide (pH region 7 to 8)
pH
The best floculation effect for natural waters is generally experienced in the
pH 6.0 to 8.0 range according to many investigators. The isoelectric point of
alumina in its hydroxide form is accepted as 7.4 pH. This corresponds to the
point of minimum solubility of aluminium hydroxide.
Coagulation tests on waters should initially use a mix of chemicals to achieve
a water pH of 7.4
Rate of Reaction
The higher the total concentration of aluminium salts within the 6.0 to 8.0 pH
range the faster pin point floc formation and floc build occurs.
At higher pH than 8.0 flocs can be produced dependant on total reactant concentration.
Other chemicals in opposition
Combinations of inorganic chemicals can be used with sodium aluminate to
achieve flocculation polymerisation in situ. A largley unexplored chemical
mechanism. Chemists investigating drinking water purification by empirical Jar
Test or pilot plant techniques need to apply sodium aluminate liquor correctly
at appropriate pH levels.
A range of inorganics - ferrics, polyalums or strong acids such as sulphate and
hydrochloric can be used to produce the same effect as aluminium sulphate or
chloride in opposition.
Comment
The representation of the coagulation reaction in the Hydrous Alumina diagram
is for a system at equilibrium and involving a fixed dose of reactants. With
materials in opposition, for example alum v aluminate, a wide range of alumina
polymer is achievable provided pH's close to neutral, are maintained.
In current full scale practise pH's are chosen to achieve particular results,
for example below 7.0 may be used, because it is claimed to achieve colour
removal. The availability of sodium aluminate liquor creates opportunities for
beneficial changes to be made in pH. The material can achieve higher pH's which
are still in the coagulation range pH 7.4 to 8.0 thus giving pH's which are
'buffered' when aluminate is applied. This is a special feature not available
to other coagulants, organic or inorganic.
In working to a specific pH the ratio of alum to SAL is adjusted so that the pH
required is achieved while maintaining the same total dose of in situ polyaluminium
floc.
Chemical Balances
The reaction between alum (8%) and sodium aluminate liquor (20%) requires an
Alum to Aluminate ratio of 1.8 to 1.0 by weight
· In tonnes - 1.8 tonne Alum to 1.0 tonne Aluminate · Alumina content - Alum
(8% soln) - contains - 0.144 tonnes alumina - Aluminate (20% soln) - contains -
0.200 tonnes alumina Total = 0.344 tonnes alumina
This total of 0.344 tonne is all active alumina and inclusive in the
flocculation process.
For other chemicals it is suggested determination of the relative amounts
should be determined by titration or contact Universal Chemicals
NB Acidic materials should be of the strong acid type. Weak acids and
barcarbonates do not give precise control due to pH spread. (strong acid - weak
base theory)
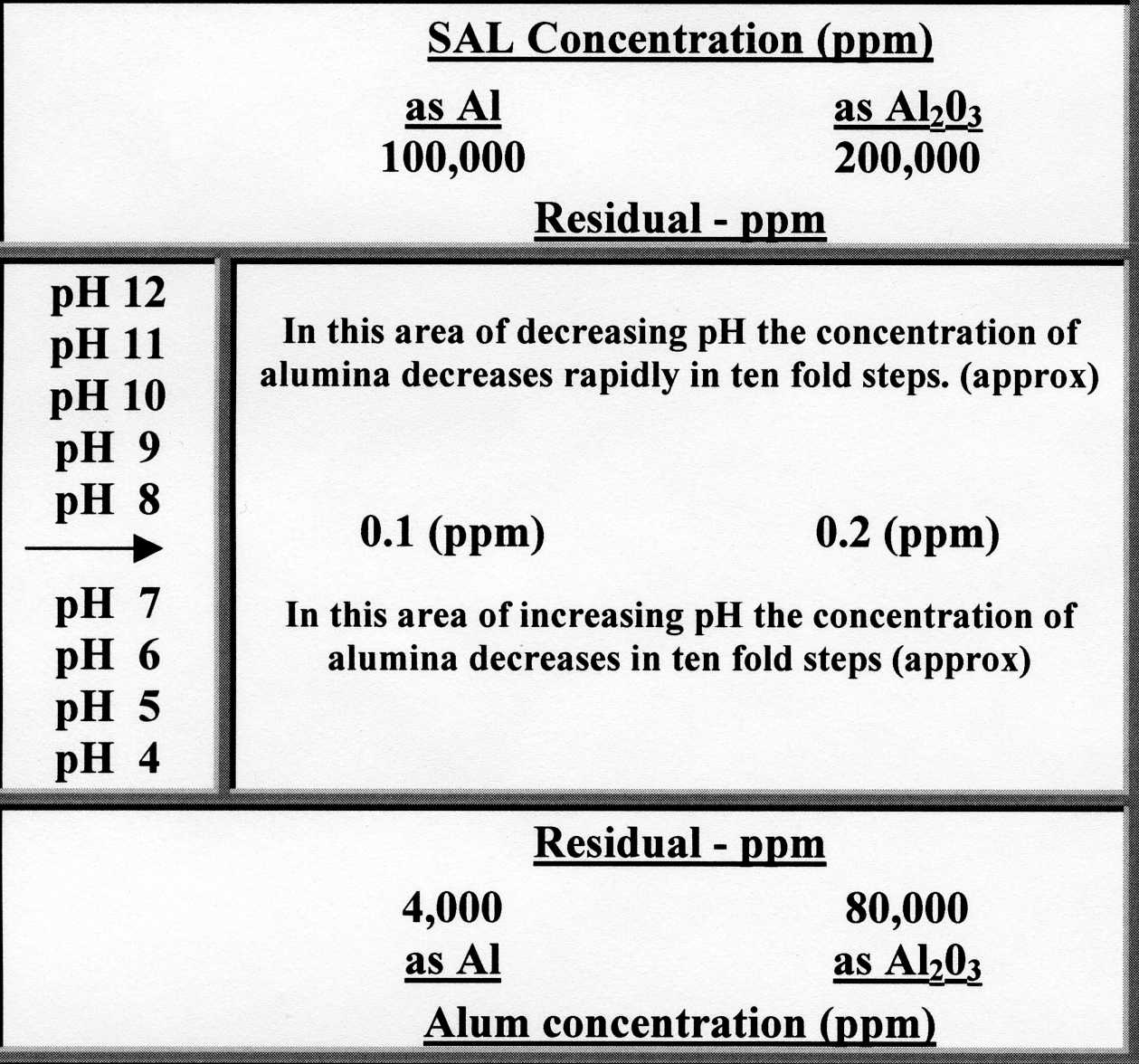
The above gives an approximation of the solubility of alumina species in water
against pH, also the very low polyhydroxy alumina level in solution with
neutral coagulation practise.
